|
Tooth Wear, Niche Differentiation and Stickleback Speciation over Evolutionary Timescales Understanding the ecological controls on the origin of new species is central to understanding evolution, but it is becoming clear there are also implications for conservation biology and the evaluation of how organisms respond to environmental change1-3. Sticklebacks are important model organisms in addressing questions of evolution and ecology (e.g. refs 4-7) and some of the most exciting and widely cited recent work on the ecological controls on speciation has focussed on these fish (e.g. refs 8, 9). In Canadian lakes, three-spine sticklebacks (Gasterosteus aculeatus species complex) occur as two coexisting trophic forms (species): "limnetics" (feed mainly on plankton in open waters; slim body, numerous long gill-rakers, narrow mouths) and "benthics" (forage on the lake bottom; larger, deeper body, fewer gill-rakers, wide mouths). Experimental evidence9 indicates that the differences between these two forms are the result of competition for food resources causing divergence in food gathering traits and ecological character displacement. But does competition and ecological character displacement operate over geological timescales to cause speciation and adaptive radiation? In fact, the role of competition in evolution is contentious10, 11, largely because of a fundamental problem in evolutionary biology: the difficulty of extrapolating from field or lab experimental results to the much longer periods over which new species evolve. The hypothesis that speciation was caused by ecological character displacement driven by trophic niche differentiation is particularly difficult to test in fossils because feeding cannot be observed directly. Such functional changes have to be inferred from changes in morphology, and determining whether morphological changes were caused by shifts in feeding thus becomes a circular argument. Recent work suggests that analysis of tooth wear patterns offers a way out of this impasse. Studies of extant mammals with well known feeding habits have revealed that the abrasives in food and the forces that act on enamel during feeding produce distinctive wear textures on teeth12, 13. This means that wear on fossil teeth can provide direct evidence of tooth use, food and feeding in extinct taxa. Almost all analyses of microwear have focussed on mammals, but recent work has demonstrated that the technique can reveal details of feeding in fossil and extant aquatic vertebrates14-15. In particular, preliminary work on cichlid fish reveals an extremely close correlation between feeding and tooth wear15, 16. |
|||
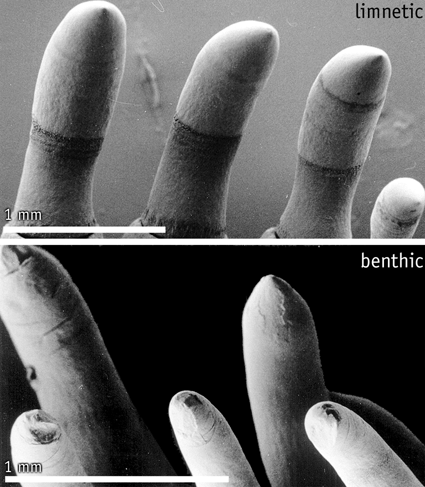 |
This project will investigate the hypothesis that tooth wear patterns in G. aculeatus allow differentiation of limnetic and benthic trophic forms in the fossil record. This will require experimental analysis of the relationship between trophic niche and tooth wear in living G. aculeatus (exploratory results [see figure] are encouraging). Tooth wear analysis will be applied to fossil members of the G. aculeatus species complex and combined with ongoing morphometric studies to investigate the hypothesis that morphological change in fossil Gasterosteus was driven by shifts in feeding habits, possibly related to niche differentiation and character displacement. The scientific significance of this project lies in the development of new methods of identifying trophic niche differentiation in aquatic vertebrates, and the rigorous testing over geological timescales of evolutionary hypotheses derived from population biology. The use of tooth wear to reveal details of feeding and diet is potentially a powerful tool, with applications across a wide range of evolutionary and ecological studies of fossil and extant organisms. | ||
|
References
|
|||